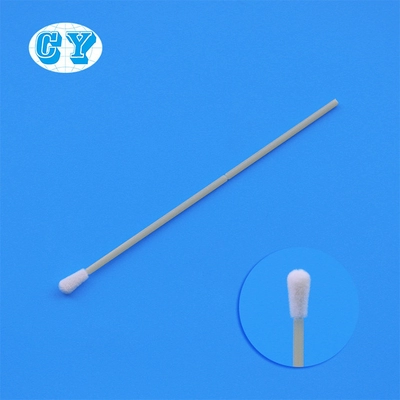
FDA Certified CHG Swabsticks Prep Skin Care Surgical Foam Head
FDA Certified Disposable CHG Prep Swab Stick Prep Skin Care Surgical Swab Stick CHG Swab
Product introduction:
CHG Prepping Products with the formulation of 2% chlorhexidine gluconate (CHG) and 70 percent isopropyl alcohol (IPA) patient preoperative skin preparation to meet the Food and Drug Administration's (FDA) strict clinical requirements for a New Drug Application (NDA), kills more bacteria than traditional iodophors or alcohol and it is thought by many clinicians to be the most exciting breakthrough in antiseptics since povidone-iodine.Chlorhexidine Gluconate the most effective antiseptic skin prep, is now available in an innovative new swab stick technology. The design of the new CHGPrep swab stick provides a significant improvement in performance over traditional swab sticks
product structure:
Cotton swab head + cotton swab holder + small bottle
Scope of application:
TOC analysis as part of cleaning validation protocol
As part of other cleaning validation protocols
Pollution source non-organic liquid sampling survey
Product advantages:
The material of the cotton
swab stick is stronger than the ordinary plastic stick, and it is easy to use.
More effective at reducing skin bacteria than other antiseptics
Exhibits a rapid & persistent bactericidal activity without irritating the skin
Active in protein-rich biomaterials
Product Usage:
It is used to apply disinfectant to the skin, mechanical wounds and instruments of the surgical or puncture site.
Total Organic Carbon (TOC) Test Procedure:
(1) Verify that the equipment is clean and that the operator is wearing overalls suitable for testing (clean gloves, face shield, gown, hair net, clean environment).
(2) Take a "clean" low TOC swab in a certified low TOC vial.
(3) Fill the vial with 40 ml of ultrapure low TOC water and seal it.
(4) Vortex the vial for 15 seconds.
(5) Fill another certified low TOC vial with 40 ml of ultrapure, low TOC water and seal. This will serve as a "blank".
(6) An instrument that runs a "control blank" dosing bottle and water level through the TOC test. The unit of measurement should be μg/L.
(7) Pass the vial with the swab inside through the TOC test device. The unit of measurement should be μg/L.
(8) Subtract the "control blank" from the swab vial to obtain the final measurement. All measurements should be in μg/L. Our goal is less than 50 μg/L.
| Brand Name |
HUACHENYANG |
| Place of Origin |
Guangdong, China |
| Material |
Other, Foam head, plastic stick |
| Solution |
2%w/v CHG+70%v/v IPA, etc |
| Product name |
Disinfection CHG Swab |
| Certificate |
CE, ISO,Free sales certificate, etc |
product instructions:
Step 1
Simply tear the pouch at the notch to reveal the Swab handle, remove the handle and apply the foam tip on the insertion site.
Step2
Twist and remove the Swab from the Pouch.
Step 3
Place the foam flat side down on the treatment area.


 icleanswabs
icleanswabs