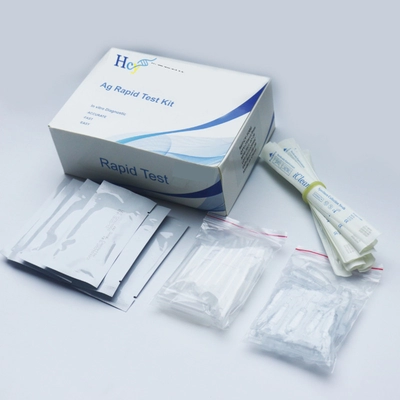
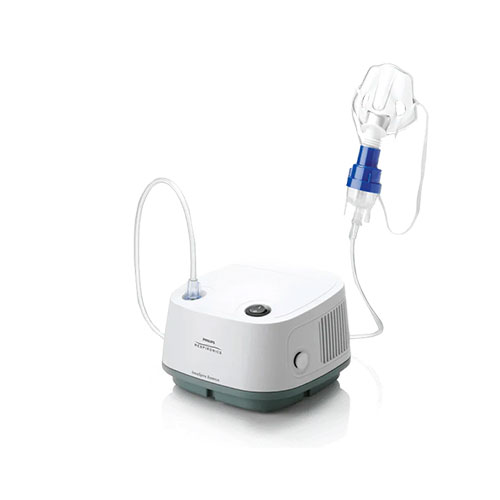
SARS-CoV-2 IgG IgM Rapid Test Kits COVID-19 Antigen Rapid Detection Kit
SARS-CoV-2 Rapid Detection IgG/IgM Rapid Detection Kit COVID-19 Antigen Rapid Detection Kit
product description:
The COVID-19 Antigen Detection Kit (Transverse Chromatography) is an immunochromatographic method for the rapid, qualitative detection of SARS-CoV-2 in nasopharyngeal (NP) and nasal (NS) swab samples, either directly or after swab collection. 2 nucleocapsid protein antigens. Added to viral transmission media by individuals suspected of having COVID-19 by a healthcare provider, testing is limited to accredited laboratories that meet requirements to perform moderate, high, or exempt complexity testing. The test is authorized for use at the point of care (POC), which is an inpatient care setting operating on the basis of a certificate of exemption, certificate of compliance, or certificate of accreditation. This test is used to identify SARS-CoV-2 nucleocapsid antigens that are commonly detected in upper respiratory tract samples during the acute phase of infection. A positive result indicates the presence of viral antigens, but clinical correlation with the patient's medical history and other diagnostic information is necessary to determine infection status, and a positive result does not rule out bacterial infection or co-infection with other viruses.
Notice:
Negative results should be considered presumptive, do not exclude SARS-CoV-2 infection, and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in conjunction with the patient's recent exposure, medical history, and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed by molecular analysis, if necessary, for patient management.
| Place of Origin |
Guangdong, China |
| Brand Name |
HUACHENYANG |
| Model Number |
CY-F006-AG25 |
| Accessory 2 |
Sampling swab |
| Place of production |
China |
| Quality Certification |
CE |
Product Usage:
This assay is used for the direct and qualitative detection of SARS-CoV-2 antigens in nasopharyngeal and oropharyngeal secretion specimens. Provides clinical results in 15 to 30 minutes, and visual interpretation of results requires no special equipment.
Product Instructions:
Sample Collection and Preparation To prepare for testing:
Remove one extraction tube and one COVID-19 antigen detection kit (transverse chromatography) cartridge from the foil bag immediately before testing
Label test cartridges and extraction tubes for each sample to be tested. Place the labeled extraction tubes on the rack in the designated area of the work area. See Figure 1.
Nasal swab specimen collection:
When collecting a nasal swab sample, carefully insert the swab (provided in the kit) into the nostril with the most secretion on visual inspection. With gentle rotation, push the swab until it encounters resistance at the level of the turbinate (approximately 2.0-2.5 cm or nearly 1 inch into the nostril). Roll the swab around the nose wall about 5 times and remove it from the nostril.
Sample Preparation:
Unscrew the cap of the sample extraction buffer bottle, squeeze the sample extraction buffer bottle, and add the sample extraction buffer to the extraction tube. (See Figure 2) Immediately after specimen collection, immerse the swab in the sample extraction buffer. (See Figure 3) To allow the extracted sample to fully penetrate the buffer of the swab, rotate the swab against the tube wall repeatedly for 10 seconds, then hold the tube with your fingers (the tube wall is semi-solid) and squeeze and wipe Several times, slowly remove the swab from the tube at the same time. (See Figure 4) The purpose of squeezing the swab against the tube wall is important because it keeps the liquid-containing specimen as much as possible in the tube. After removing the swab and discarding it in the biohazard waste container, tie the nozzle (provided in the kit) to the top of the extraction tube and gently shake the tube to thoroughly mix the liquid inside, see Figure 5.
Interpretation of results:
Positive Test Result: Visible color lines appear at both the "T" and "C" sites, or a darker color if the "T" site is tested.
Negative test result: Only the "C" reference is colored, the "T" position is blank.
Invalid result: If "C" is empty and no color is displayed, the test result is invalid and the sample needs to be re-tested.
Features:
Sensitivity: 82.14% (23/28), compared with nucleic acid amplification tests, confirmed positive cases.
Specificity: 99.45% (180/181), negative cases were also confirmed by nucleic acid amplification test.


 icleanswabs
icleanswabs