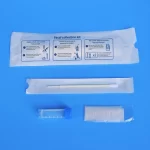
ISO13485 NMPA Fecal Collection Kit Individually Packaged Class I
Anal Swab Fecal Collection Kit Individually Packaged Disposable Sampler Sample Collection Kit
Product advantages:
With excellent sample collection and release capabilities, it can quickly adsorb small samples with high release efficiency. The duration of positive nucleic acid detection in anal swabs is longer than that in oral and pharyngeal swabs, which can supplement the detection loopholes of oral and pharyngeal swabs to prevent missed diagnosis. In the past oral and throat swab tests, samples were taken through the oral cavity or nasal cavity, which was an uncomfortable experience for the subjects. After sampling, some people would experience nausea, vomiting, nausea, etc., while The anal swab test will not have the above situation, but it will test the psychological endurance of the subjects. Anal swabs have a higher test duration and theoretically more accurately test samples
Product Usage:
For sample collection, transportation and storage, etc.
Product Instructions:
1. Take out the sampling swab, do not touch other parts or other places (so as not to contaminate the swab).
2. Gently insert the swab 3-5 cm into the anus.
3. Scrape a sufficient number of samples directly with a sampling rod, and the ratio of sample to preservation solution is 0.01-0.25:1.
4. Use the swab in the anus for 4~5 turns and then gently pull out the swab.
5. Put the sampling swab into the test tube containing the preservation solution, and quickly stir about 10 times to elute the sample on the swab. Then push the sampling swab head from the tail and tighten the cap
Sample requirements:
The collected samples must be fresh and sent for inspection in time. The pathogenic bacteria after collection should be
It should be sent to the laboratory within 48 hours at 2-8 °C. If it cannot be sent to the laboratory within 48 hours, it should be stored at -70 °C or below. The pathogenic bacteria samples should be inoculated and separated as soon as possible after they are sent to the laboratory. After inoculation and separation within 48 hours, they can be stored at 2-8 °C. If it is not inoculated and separated in time, it should be stored at -70°C or below.
Packing specification:
25 servings/pack
500 servings/box
| Model Number |
CY-90003Amies |
| Brand Name |
HUACHENYANG |
| Size |
/ |
| Instrument |
Class I |
| Validity period |
12 months |
| Place of Origin |
Guangdong, China |
Q1: Do you support OEM/ODM?
A: YES, we can prototype and customize the ideal product according to your specific application.
Q2: Do you have GMP Lab?
A: YES, we have our GMP Lab built in 2016.
Q3: Do you have any certificates?
A: YES, we have all the certificates required for export and local import custom clearance.
Q4: How long have you been in manufacturing medical consumables?
A: Established on 2008, our factory offer production, sales,delivery one-stop service.
Q5: Where is your main market located?
A: 60% in American Countries, 30% in European Countries and 10% in Middle East.


 icleanswabs
icleanswabs